Follicum Comes Back To Share New FOL005 Presentation May 2023
After a more thorough evaluation of FOL-005’s data from a phase 2 clinical trial, new management at Follicum believes that its drug candidate has a place in the treatment landscape for androgenic alopecia and they want to explain why.
FOL-005 Phase 2 Results Revisited
In February 2022, Follicum AB was acquired by Coegin Pharma, a biotechnology company with headquarters in Sweden. Several months later, it was announced that new leadership at Follicum had completed a further analysis of FOL-005’s data from a phase 2a clinical trial in men, which revealed a previously unseen positive outcome. Recently, I had the pleasure of communicating with members of the management team at Follicum/Coegin Pharma, including CCO Kristian Lykke Fick, CMO John Zibert, and CEO Tore Duvold, about the current plans for FOL-005. In addition to their commentary, the team at Follicum provided a PDF presentation, linked below, which contains new insights into the data which FOL-005 produced in its previous phase 2a clinical trial. As we began our discussion, I was most interested in learning about how Follicum became inspired to re-examine the FOL-005 data, and exactly how it could become a resurrected drug. Below are the main takeaways from my discussion from the team at Follicum:
- The team at Coegin Pharma had a natural business and scientific inclination to analyze data from their newly acquired intellectual property, FOL-005, and another closely related peptide in development for diabetes. Upon further exploration of the FOL-005 phase 2a data, there was a pattern discovered among the trial subjects who showed a statistically significant hair growth response to the drug.
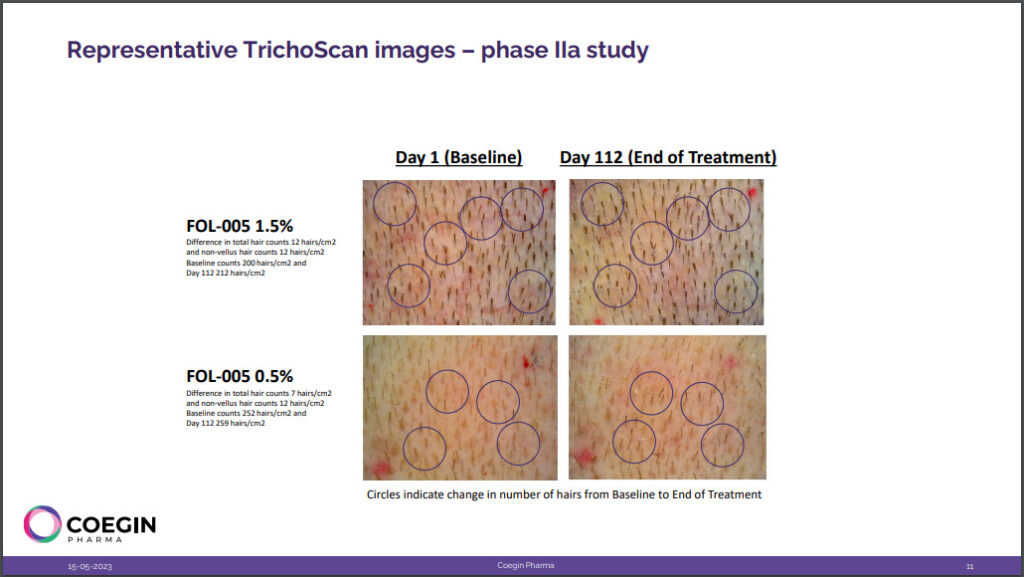
- Follicum’s new revelation was that patients with less than 255 hairs per cm2 were found to be the optimal treatment group. In its presentation, Follicum states that normal hair growth density is above 250 hairs per cm2, thus, patients with less hair growth respond better to FOL-005.
- Among the total trial participants, around 40% of the patients met the criteria of having less than 255 hairs per cm2, and the average response rate among that sub-group was a ~12 hair increase per cm2 from baseline.
- With this understanding, the team at Follicum is now pursuing a phase 2b trial for FOL-005 and is raising $10-$15M USD towards that effort.
- The team believes that a 6 – 9 month trial could produce even more favorable hair growth results and would need to conduct a 6 month preclinical safety study prior to initiating a longer phase 2b study, as FOL-005 was previously tested in safety studies up to 4 months.
Click here to view Follicum’s latest exclusive presentation on FOL-005
Beyond these main topics, Follicum also has interests in trialing FOL-005 in Asian territories and/or among a cohort of female patients.
Neuropilin-1 Receptor Agonist
One potentially advantageous facet of FOL-005, according to Follicum, is its mechanism of action. FOL-005 binds to the neuropilin-1 receptor (NRP-1) and produces a stimulating or agonistic effect. This lands outside of more typical attempts to manipulate hair growth involving the androgen pathway which can lead to sexual side effects and are usually unsuitable for women. Follicum’s website has a page dedicated to explaining its research in further detail.
In summary, new evidence has been shared which shows hope for FOL-005 in hair growth, and Follicum is actively seeking business collaboration to pursue its development of the drug. Recently, an androgen receptor-inhibitor drug showed a 10 hair increase in a US phase 2 trial and that drug is heading towards a phase 3 trial. Although the treatment group samples from each trial are not exactly the same, those androgen receptor inhibitor results shine a new perspective on the ability of FOL-005 to create a 12 hair increase per cm2 in male AGA. Hopefully, we get to see how FOL-005 fares in a longer phase 2b study in the future.
Posted in Coegin Pharma, Follicum