Hair Growth Industry Happenings Spring 2023
As we head into Q2 2023, the hair growth treatment industry continues to develop and make progress in various ways.
Kintor Begins Phase 2 Trial For GT20029 Drug
This news appeared one day following the publishing of this article. Kintor Pharmaceutical of China has dosed the first patient in a trial which will comprise 180 male subjects in a multi-center, placebo-controlled study. The trial dosing period for each subject will last 12 weeks. More details can be found on the Kintor website.
Hope Medicine Approved For Phase 2 In China
On March 17, 2023, the corporate blog page of Hope Medicine announced the company has received regulatory approval to begin a phase 2 clinical trial for its drug HMI-115 in androgenetic alopecia in China. The approval comes from the National Medical Products Administration (NMPA) of China. There is no estimated timeline for the trial to begin, according to the release, other than Hope’s new CEO Mr. Chen Xi stating that the company will focus on beginning the trial in the future. Likely, though not necessarily, the company will wait for its current phase 1b trial in Australia to conclude before beginning a phase 2 in China.
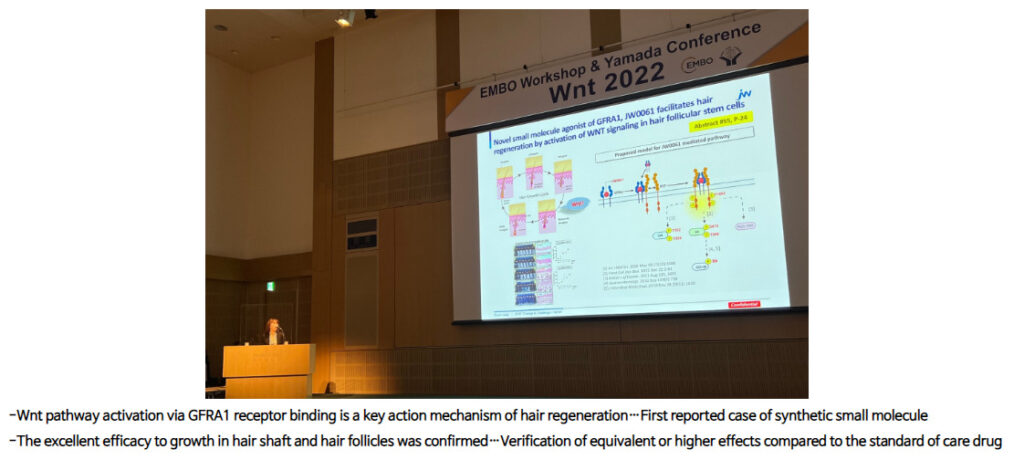
JW Pharmaceutical Announces Patent Approval For Wnt Hair Loss Drug
JW Pharmaceutical, a Korean biotech company which specializes in the Wnt pathway, recently announced the granting of a patent for their hair regeneration drug JW0061 in Russia. The news was announced in the Korea IT Times on March 27, 2023. The news is a positive indication for the development of JW0061 and JW Pharma stated they aim to enter a clinical trial for the drug in the first half of 2024. JW0061 binds to the GFRA1 protein to stimulate hair growth, which represents a new mechanistic approach for the hair growth biotech industry. In an announcement on their website, JW Pharma says that their drug activates the Wnt pathway in hair follicle stem cells and that they have already begun research with a “a key opinion leader in the dermatology field in the USA.” Because the Wnt pathway is involved, I believe JW0061 is one of the more interesting new drugs on the horizon.
Rion Aesthetics Working On Hair Growth
A subsidiary of a biotech company founded by Mayo Clinic doctors to harness the regenerative power of exosomes, Rion Aesthetics, has an interest in hair growth. The Rion Aesthetics website writes “We have investigational studies currently underway for hair restoration and facial wrinkles for potential FDA indications.” The active ingredient for RA’s products is called PEP, which stands for Purified Exosome Product. More can be learned about PEP on the parent company Rion’s website. A representative at Rion Aesthetics was kind enough to share some insight into the company’s plans for hair growth with Follicle Thought, which include a topical hair/scalp cosmeceutical which could be released by the end of 2023 and an injectable version which may enter FDA trials this year as well. This news was originally shared on the Updates page and can be found there. With all of the several new cosmeceuticals on the horizon we may finally have some useful options beyond the status quo, though this is not guaranteed until results are proven.
Line Items
- A new drug discovery company based in India called INOVAUGMET has apparently discovered a new molecule aimed at treating hair loss. The company’s website which now shows a “Coming Soon” page previously displayed information, including a pipeline chart, over the past several months. For now, their Linkedin profile is still up, so keep an eye on their website in the coming months.
- The number of companies working quietly on hair growth/loss is more than we’d think, especially in Asia. The company FromBIO is another example of a company based in Korea which describes working on new therapies for alopecia including a new drug and potentially a stem cell technology. While we never know which company will become a winner, or how involved their research is, the indication of interest among many biotech companies is encouraging.
- Orient Bio is yet another company not discussed online which has a hair growth candidate, OND-1, in its portfolio. Based on information provided on the company’s website, OND-1 appears to be a derivative of cyclosporine A. The Orient Bio website mentions items such as: “has a different mechanism from existing ‘Propecia’ and ‘Minoxidil’ drugs….”very low renal toxicity compared to CsA”….”Currently, we are preparing for phase 2 clinical trials after receiving approval from the US FDA for clinical trials.” Note, it is unclear how long Orient Bio has been considering the pursuit of OND-1 and what the company’s actual current plans are.
- CosmeRNA is likely to begin selling through Amazon Europe within the next 4-6 weeks. There is no new information available, but there have been around 10 news articles published since the beginning of the year which claimed that fact.
- HairDAO recorded the second episode of their Hairy Matters podcast series featuring Dr. Ralf Paus, widely respected as one of the world’s top hair researchers. On Twitter recently, HairDAO mentioned that their community which raises money for hair growth research will be voting to fund new research from a pool of ~10 study candidates this month. To get involved in the HairDAO community, follow them on Twitter or join their community messageboard.
Thanks to the various readers who shared news tips.