Updates
Cures For Hair Loss 2023 - News Feed
A very good idea is to bookmark this article page in your browser or mobile browser for easy access on the latest hair loss cure and hair growth treatment news. This will allow you to check it easily for updates. If you are looking for a corresponding article from a past update, simply use the “Search” function on this site to locate the article. As always, thanks for reading and please share this information with all you feel would benefit from it!
Hope Medicine’s HMI-115 Positive Phase 1b Outcome
Positive news for those following Hope Medicine's HMI-115. The company has successfully completed a Phase 1b study in Australia, conducted in 12 male and 4 female patients with alopecia areata. The results showed positive efficacy, with HMI-115 being safe and well-tolerated. In male patients, the mean non-vellus target area hair count (TAHC) increased by 14 hairs/cm2 by the end of the trial, which was statistically significant compared to the baseline measurements. However, no results have been released yet for the female participants. These results have led to the start of recruitment for the Phase 2 study, which will be conducted in China with 180 participants.
Epibiotech Approved to Start Phase 1/2a Trials
As of December 21st, 2023, Epibiotech has been approved by the Ministry of Food and Drug Safety to start Phase 1/2a clinical trials for its EPI-001 autologous dermal papilla cell treatment to treat androgenetic alopecia. Safety will be measured in Phase 1 to set the maximum tolerated dose (MTD), and then the effectiveness of the treatment will be evaluated in the Phase 2a trial. Epibiotech plans to administer the treatment to the first patient in the first half of 2024, with completion expected in the second half of 2025.
Yuva Biosciences Enters into a Collaboration with Elevai Labs
On November 29th, 2023, ELEVAI Labs, a medical aesthetic company, announced that they had signed a licensing agreement with Yuva Biosciences, Inc. This will combine ELEVAI's human umbilical mesenchymal stem cell-derived exosome technology with Yuva's mitochondrial research in an effort to develop pharmaceuticals and cosmetics targeted toward aging hair and skin. The collaboration might allow for the development of new products to address cosmetic skin and hair concerns associated with signs of aging.
Kintor Pyrilutamide KX-826 Phase 3 Results Shared
The world's current furthest developed drug for androgenic alopecia prior to approval, Kintor's pyrilutamide aka KX-826, has completed its phase 3 trial for men in China. A non-detailed explanation of the trial's data was sent out by Kintor Pharmaceutical on November 27, 2023. In short, pyrilutamide showed overall safety in the trial with no serious adverse events reported, the drug also showed an increase in target area hair count (TAHC) in the treatment group compared to their baseline numbers, and it also showed improvement in TAHC over placebo during all treatment visits of the trial, though they were not statistically significant. Therefore, overall KX-826 did not show a statistically significant improvement in TAHC compared to placebo. The future of pyrilutamide for AGA is now uncertain.
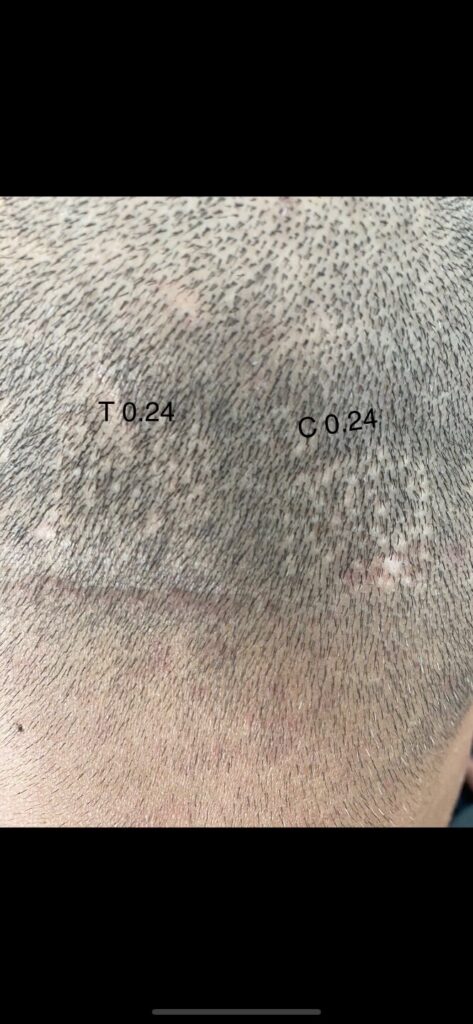
Dr. Barghouthi Verteporfin Study Updated
The groundbreaking verteporfin study done by Dr. Barghouthi of the Vertex Hair Clinic in Jordan has recently been updated with new photos. For the newcomers, the study involved injecting the drug verteporfin into FUE donor area extraction sites, i.e. wounded scalp skin. Based on preclinical research at Stanford, it is believed that verteporfin has the potential to decrease or eliminate scarring, and/or regenerate hair follicles. Below is the least impressive of the recent photos shared by Dr. Barghouthi. Check out the original documented post on Follicle Thought to see the higher concentration of 0.4mg injections ~1.5 years post surgery.
Veradermics Doses First Patient With New Oral Hair Loss Drug
A fascinating new dermatology startup, Veradermics, announced today the dosing of its first patient in a phase 2 clinical trial for pattern hair loss. What makes this news especially exciting is that Veradermics' drug, for now referred to as VDPHL, is an oral medication. Further, it's a non-hormonal drug, which means it does not act on androgen hormones, such as DHT, like the drugs finasteride or dutasteride do. This means that users will likely not have to worry about sexual-related side effects. While information about VDPHL is limited at this time, Veradermics describes it as a "proprietary, non-hormonal oral treatment being developed for pattern hair loss." Stay tuned for further information about this drug program here on Follicle Thought.
HMI-115 Starts Phase 2 Clinical Trial In China
As the title says, HMI-115 is now at the phase 2 clinical trial stage in China. The placebo-controlled trial will include a 6 month treatment period for its participants. For now, this news seems to be the capstone of a quite active and positive year for hair loss treatment companies. To read the full story, check our last article on Hope Medicine.
OrganTech Takashi Kondo Interview
Follicle Thought was fortunate to receive exclusive information from OrganTech, the company developing Dr. Tsuji's hair cloning technology, this past week. An interview was conducted with OrganTech's CEO Takashi Kondo which shed light on the company's latest clinical trial projections, upcoming plans for funding, and more. Head to the Articles page, or use the Search bar on any page to read the full story.
JW Pharmaceutical Collaborates With Microneedle Technology Company
JW Pharmaceutical of Korea, a biotech company developing an extremely interesting Wnt targeting drug for hair regrowth, has announced a business collaboration with Theraject Asia. Under the agreement, JW Pharmaceutical and Theraject Asia will develop a microneedle-based hair regrowth treatment, according to Korean press. JW's drug candidate JW0061 will likely be the active pharmaceutical ingredient in the treatment. Microneedle treatments are essentially adhesive patches containing numerous needles of an extremely fine width, which deliver API's into the bloodstream or scalp. They are thought to be next-generation drug delivery technologies and are still in early stage development for practical clinical use.
Triple Hair’s TH-07 Licensed By South Korean Pharma Co.
Triple Hair of Canada announced yesterday that their flagship drug candidate TH-07 has been licensed by Mother's Pharmaceutical of Korea. TH-07 is a combinatorial topical pharmaceutical product for hair regrowth comprising minoxidil, finasteride, and latanoprost. With the hair growth capability of those three ingredients well documented (especially with minoxidil and finasteride), the product stands to be a logical, albeit familiar, prescription treatment success. For the North American markets, TH-07 is still preparing to enter a pivotal phase 3 clinical trial, and the company's website is currently recruiting male patients.
The recent licensing agreement with Mother's Pharmaceutical appears to have given Triple Hair ~$2.75M in an up-front payment, with potential milestone payments as part of the deal. This capital will likely be put towards the initiation of a phase 3 clinical trial in North America to seek widespread regulatory approval as a safe and effective treatment for hair loss.