Nektar Therapeutics Launches a Phase 2b Trial for Alopecia Areata
This week, there is interesting news for those with alopecia areata. Nektar Therapeutics has announced that it will launch a Phase 2b trial for its drug rezpegaldesleukin (REZPEG), which will be assessed in individuals suffering from severe to very severe alopecia areata (AA).
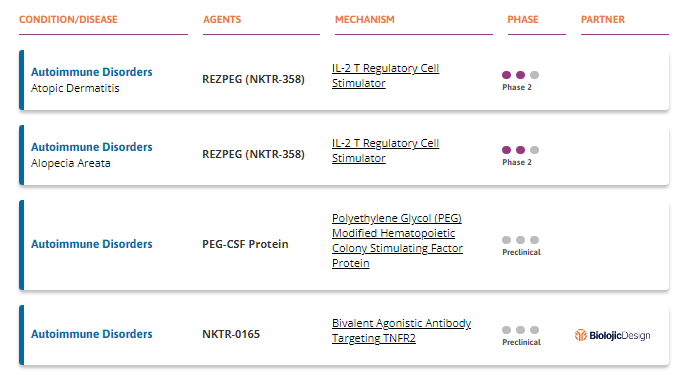
Nektar Therapeutics is a biotech firm with several indications in the pipeline for treating autoimmune diseases. Its products target cytokine pathways and other mechanisms to modulate the immune system.
What is REZPEG?
Rezpegaldesleukin (REZPEG or NKTR-358) is a regulatory T-cell (Treg) stimulator developed to correct immune system imbalances implicated in autoimmune and chronic inflammatory conditions. The rationale behind the development of REZPEG is the recognition that inadequate levels and functionality of Tregs, which are essential for regulating immune responses, can contribute to the development of autoimmune and inflammatory diseases. Nektar Therapeutics aims to address this by targeting the IL-2 receptor complex, specifically encouraging the growth of Treg cells without activating the cytotoxic CD8+ T and CD4+ T cells responsible for driving autoimmune disease. This mechanism is intended to suppress the activity of disease-causing T cells and reestablish the body’s natural self-tolerance processes.
REZPEG is under development as a self-administered injection.
Launching into Phase 2
Nektar’s Phase 2b study has been designed as a global, randomized, double-blind, placebo-controlled trial. Its goal is to determine the safety and effectiveness of REZPEG in 84 participants over 36 weeks. Two dosing regimens of REZPEG will be compared to a placebo, with an additional 24-week follow-up to assess treatment durability. The trial’s primary outcome will focus on the mean percent improvement in the Severity of Alopecia Tool (SALT) score at week 36 and secondary measures, including the proportion of participants achieving significant SALT score reductions. Initial results are anticipated in the first half of 2023.
Along with AA, REZPEG is under evaluation for atopic dermatitis. An abstract of the study’s results presented at EADV 2023 can be found here.
Reflections
Two recent FDA-approved treatments for alopecia areata are baricitinib and ritlecitinib. These differ from REZPEG in that they are Janus kinase (JAK) inhibitors. While both are immodulatory therapies, they serve different functions. Treg stimulators aim to restore immune tolerance by enhancing the function of regulatory T cells, potentially preventing the immune system from attacking hair follicles. Conversely, JAK inhibitors target inflammatory signaling pathways involved in the disease, reducing immune-mediated hair follicle destruction.
We have previously covered other research examining the modulation of Treg migration and activity here (although that research was conducted in mice). We look forward to reviewing the study’s results.
Let us know what you think in the comments below.
Posted in Hair Growth Treatment


Hi
Did you see the research out of Japan on Keratin microspheres as a treatment for hair loss?
Yes, and, with sufficient funding, it will be available in 5 years. 😉